New Extension - Internal Audit Checklist for ISO 13485:2016
There are few standards that have higher influence on the daily workday than ISO 13485. Most processes used by a Medical Device manufacturer are mentioned and regulated by this ISO standard.
If you want to check how well your organisation fulfils the ISO 13485, you can use this new Aligned Elements Extension in your Aligned Elements instance.

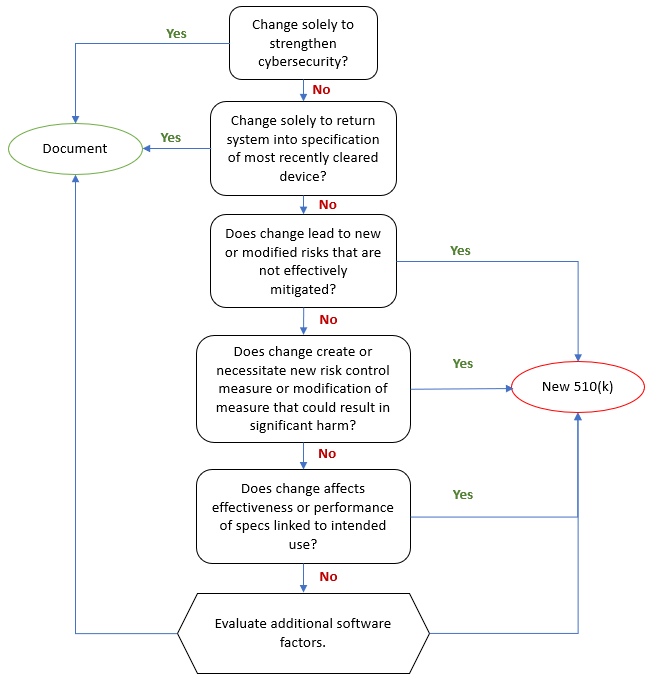
This checklist extension covers key clauses in the sections:
4 - Quality management system
5 - Management responsibility
6 - Resource management
7 - Product realization
8 - Measurement, analysis, and improvement
With this checklist it is easier for you to assess what you have completed and what the remaining items are. Use the standard Aligned Elements functions to collaborate, analyse and report your results.
How does the ISO 13485 Checklist work?
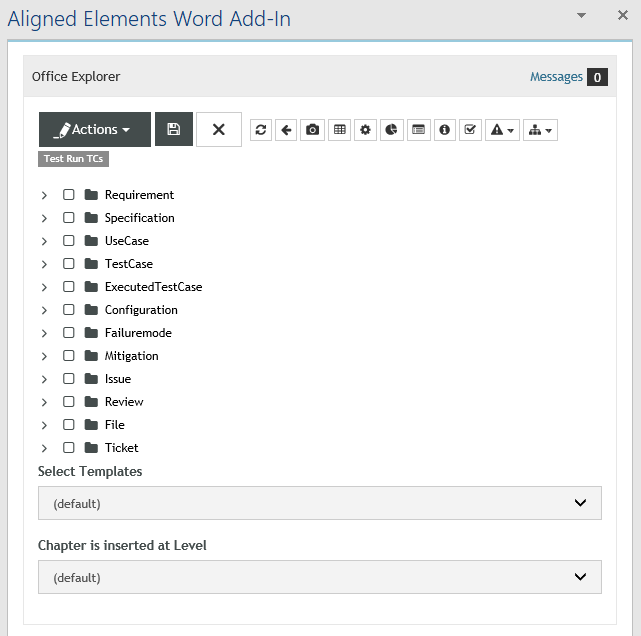
The ISO 13485 Checklist Extension consists of:
- A new template called "Checklist Item" incl. a Word Template for this type
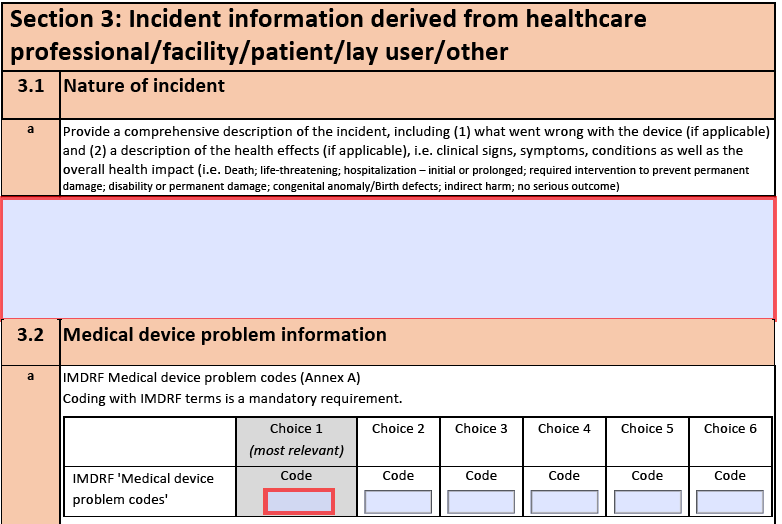
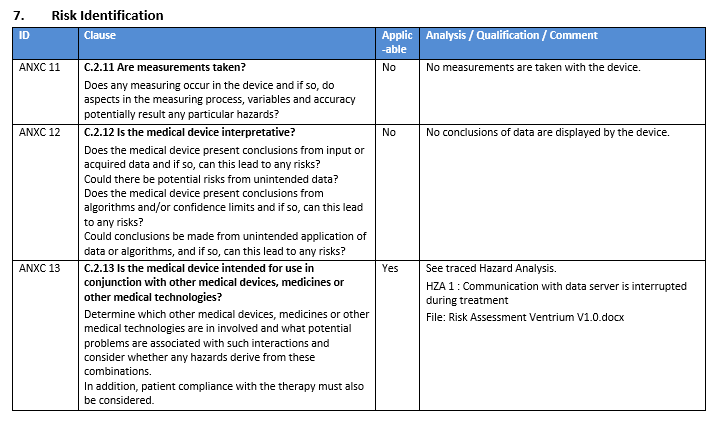
- 143 importable checklist items that cover chapter 4 to 8 in ISO 13485:2016
- Each item contains a control question, an assessment of the question and a placeholder for adding evidence for the assessment
Use this checklist to get a properly controlled and document proof of your fulfilment of ISO 13485.
Note! This Internal Audit Checklist for ISO 13485 only works in Aligned Elements.