New: A IEC 60601-1 Risk Assessment Checklist
The IEC/ISO 60601-1 "Medical electrical equipment" is the cornerstone document addressing many of the risks associated with electrical medical equipment.
The standard covers safety and performance requirements of medical electrical equipment and public health authorities in many countries recognize it as a pre-requisite for market access. The standard is notorious for its depth and complexity and many manufacturers experience the task of ensuring compliance as challenging.
The safety testing, certification, and global market access approvals are done for IEC 60601-1 shall be conducted by an accredited Testing Lab. The manufacturer's collaboration with the Testing Lab is essential for a smooth and swift approval.
As of the 3rd edition of IEC 60601-1, a large number of risk management references were introduced in the standard. The Test Laboratory will request the manufacturer to demonstrate how the product's risk assessment covers the risks items stipulated in IEC 60601-1.
Poor preparation of this step can result in a delay in the certification process, requiring an inordinate amount of time during the initial testing phase to correct the risk management files.
To facilitate this step, Aligned has developed an integrated assessment method in Aligned Elements, that assists the manufacturer in demonstrating compliance with these risks.
By assessing and connecting the IEC 60601-1 risk requirements with the product risk assessment already existing in Aligned Elements, a compliance assessment document can be automatically generated and presented to the Testing Lab.
Aligned Elements IEC/ISO 60601-1 Risk Assessment Checklist - How is it done
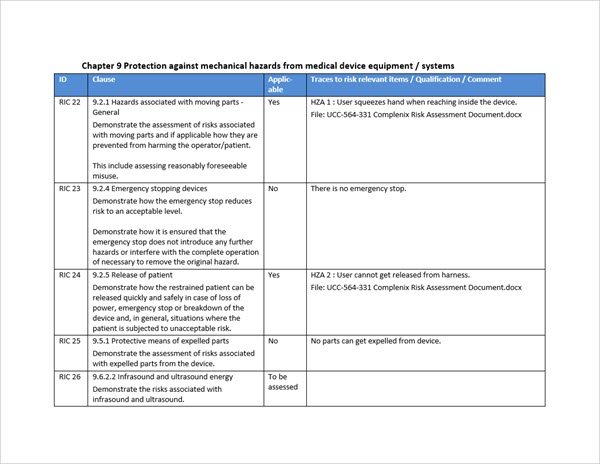
- The checklist contains approx. 80 risk checklist items from IEC 60601-1 which are imported into Aligned Elements.
- Each IEC 60601-1 risk checklist item contains the clause reference, the demonstration requirement, explanations, and examples of what the risk intends to cover, all to facilitate the identification of the corresponding risk in the manufacturer's risk assessment, already located in Aligned Elements.
- The manufacturer addresses each risk requirement, deeming it either as "not applicable" for his product (including providing a qualification for the answer), or applicable and then tracing the risk requirement to the corresponding existing risks in his own risk assessment.
- When completed, a Compliance Assessment Word Report is generated and can be presented to the Test Laboratory.
- With this compliance report, the Test Laboratory representative can quickly assess your IEC 60601-1 risk related compliance level
The benefit of the Aligned Elements IEC/ISO 60601-1 Risk Assessment Checklist is a massive reduction of time spent at the Testing Lab by leveraging your existing documentation!
How we developed the Aligned Elements IEC/ISO 60601-1 Risk Assessment Checklist
The Aligned Element IEC 60601-1 Risk Assessment Checklist has been developed in collaboration with former Eurofins Electrosuisse Test Laboratory Manager Karim Bader, currently serving in the swiss national working group CES/TK 62 for "Elektrische Apparate in medizinischer Anwendung" contributing to the development of the international standard IEC 60601-1.
Experts from Aligned will assist you in integrating the checklist into your current configuration and demonstrate its use.
If there is a need to further explain the IEC 60601-1 risk management requirements and identify findings that can be fixed, Mr. Karim Bader, an expert in this field is available to deliver the knowledge and confidence to ensure that your product will be certified without delay.
Contact