Design Controls according to IEC 82304
{fastsocialshare}
When you think about standards for medical device software, IEC 62304 is probably the first one that comes to mind. If you develop software for medical devices, this is definitely the standard you should look into.
Less known is IEC 62304's bigger brother, the IEC 82304 "Health software — Part 1: General requirements for product safety" which expands on the IEC 62304.
So what are the main differences? When shall I use which standard?
Differences between IEC 62304 and IEC 82304
Scope
IEC 82304 targets a larger range of device types than IEC 62304. IEC 82304-1 targets any kind of software, which directly or indirectly has an effect on health, known as "health software" in the standard.
In contrast, IEC 62304 only targets software with medical intended use.
Examples of device types that are in the scope IEC 82304 but not IEC 62304:
- Radiology Information Systems
- Prescription Management Systems
- Laboratory Information Management Systems
- Mobile Apps (not defined as FDA Mobile Medical Apps)
Standalone
IEC 82304 is intended for standalone software and not as IEC 62304, software embedded in medical devices or embedded in devices with specific hardware.
Software running on PCs, Server or Mobile Devices on a general purpose Operating System are in the scope of IEC 82304.
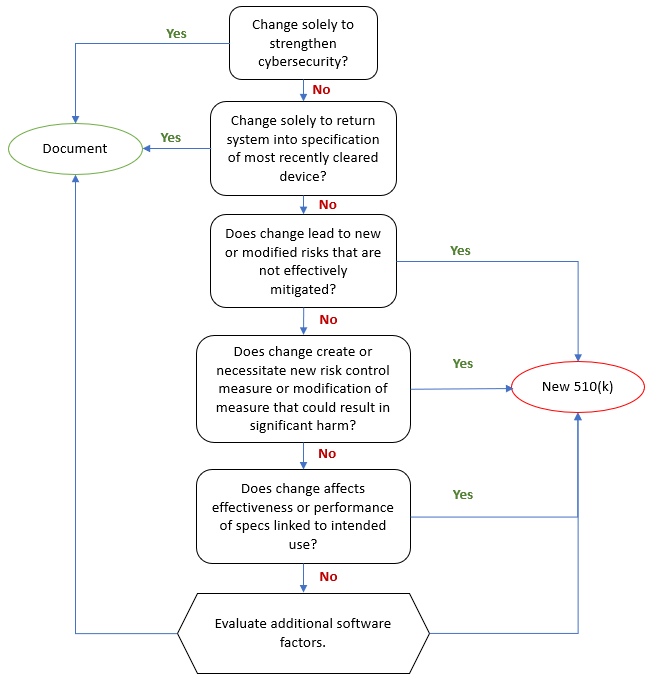
Product Life Cycle Stages and Design Control Levels
As mentioned, IEC 82304 expands on IEC 62304 and addresses both additional Design Controls and requirements on expanded parts of the Product Life Cycle.
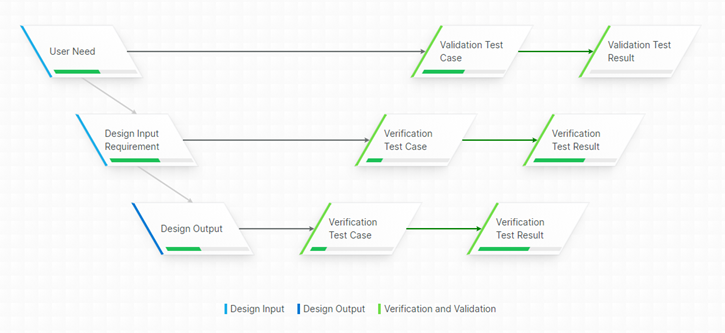
It includes Product Use Requirements as well as Product Validation Plans and Reports. It specifies requirements on Product Identification and Instructions For Use as well as Post-market activities.
IEC 82304 references IEC 62304 for software development and maintenance, i.e. on a software level.
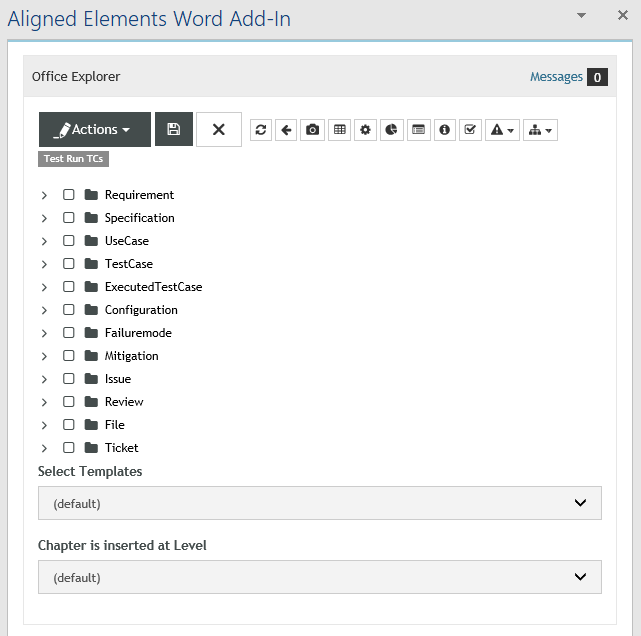
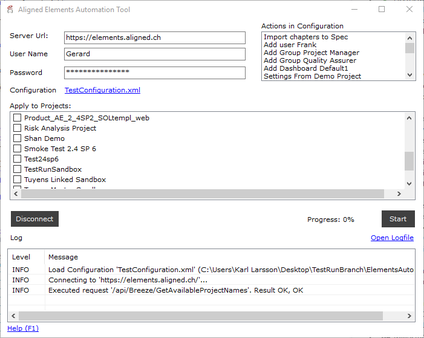
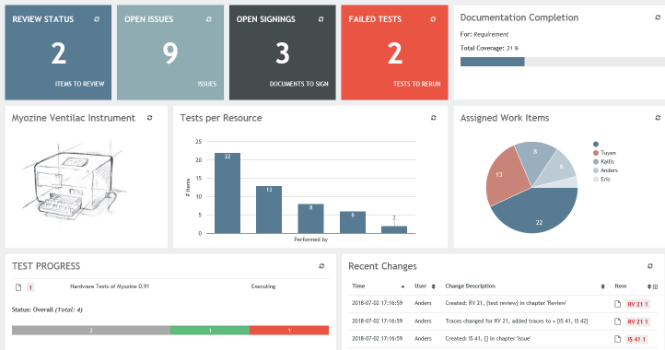
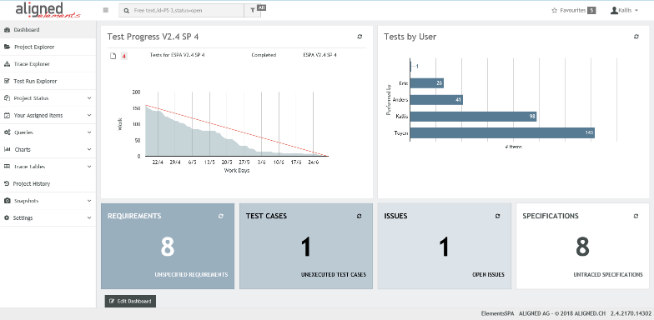
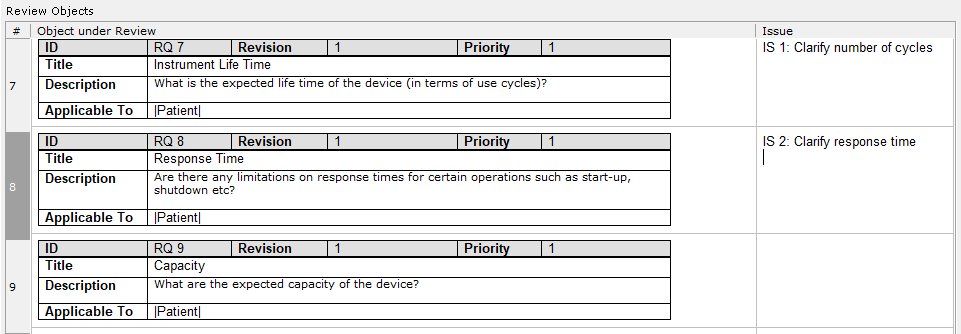
Aligned Elements IEC 82304 Configuration
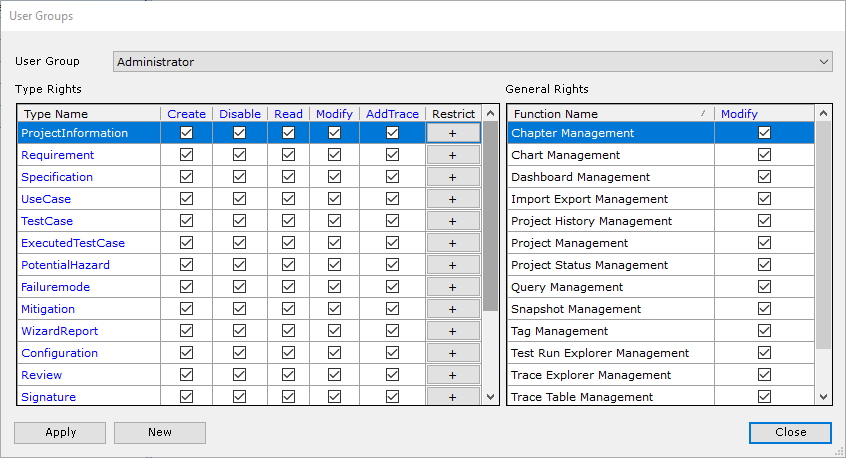
The Aligned Elements IEC 82304 configuration is a superset of the IEC 62304 configuration. It expands the IEC 62304 configuration with Product Use Requirements and Product Validation Tests. It has been tuned to automatically take care of most of the involved quality checks, making sure that the required tasks and actions are sufficiently covered.
The Aligned Elements IEC 82304 configuration contains:
- Pre-configured templates using IEC 82304 standard naming conventions
- Software Safety Classification automatically based on risk analysis results
- Numerous quality checks for consistency verification
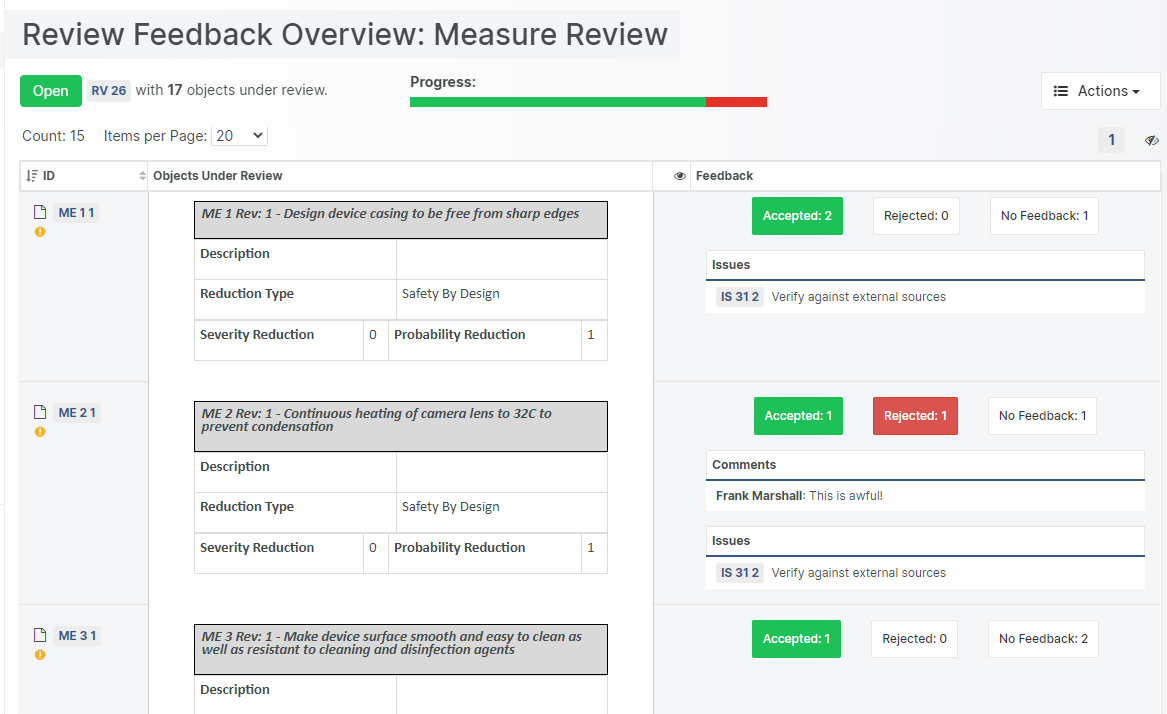
- Pre-configured Reviews and checkpoints according to IEC 82304 and IEC 62304 stipulations
- Pre-configured Trace Tables based on the IEC 82304 and IEC 62304 requirements
- A set of document templates being a great starting point for your documentation
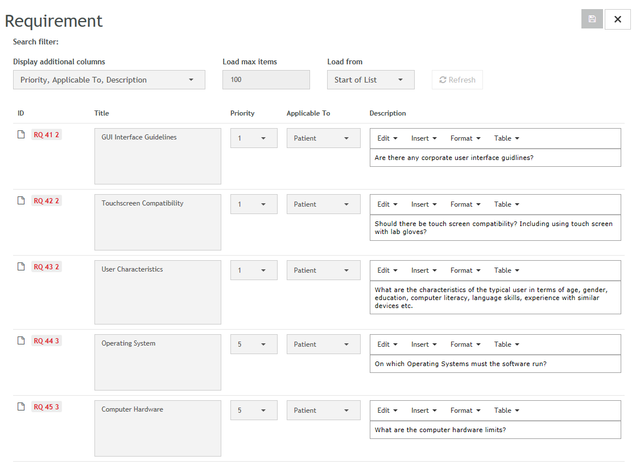
- 48 importable Product Use Requirements for Accompanying Documentation from IEC 82304
The Aligned Elements IEC 82304 supports documentation management of:
- Product Use Requirements
- Product Validation Tests and Results
- System and Software Requirements
- Software Architecture building blocks(Software Items, Units, SOUPs, and segregations)
- Risk Management using a Preliminary Hazard Analysis technique (listed in ISO 14971)
- Software Verification (Unit, Integration and System testing)
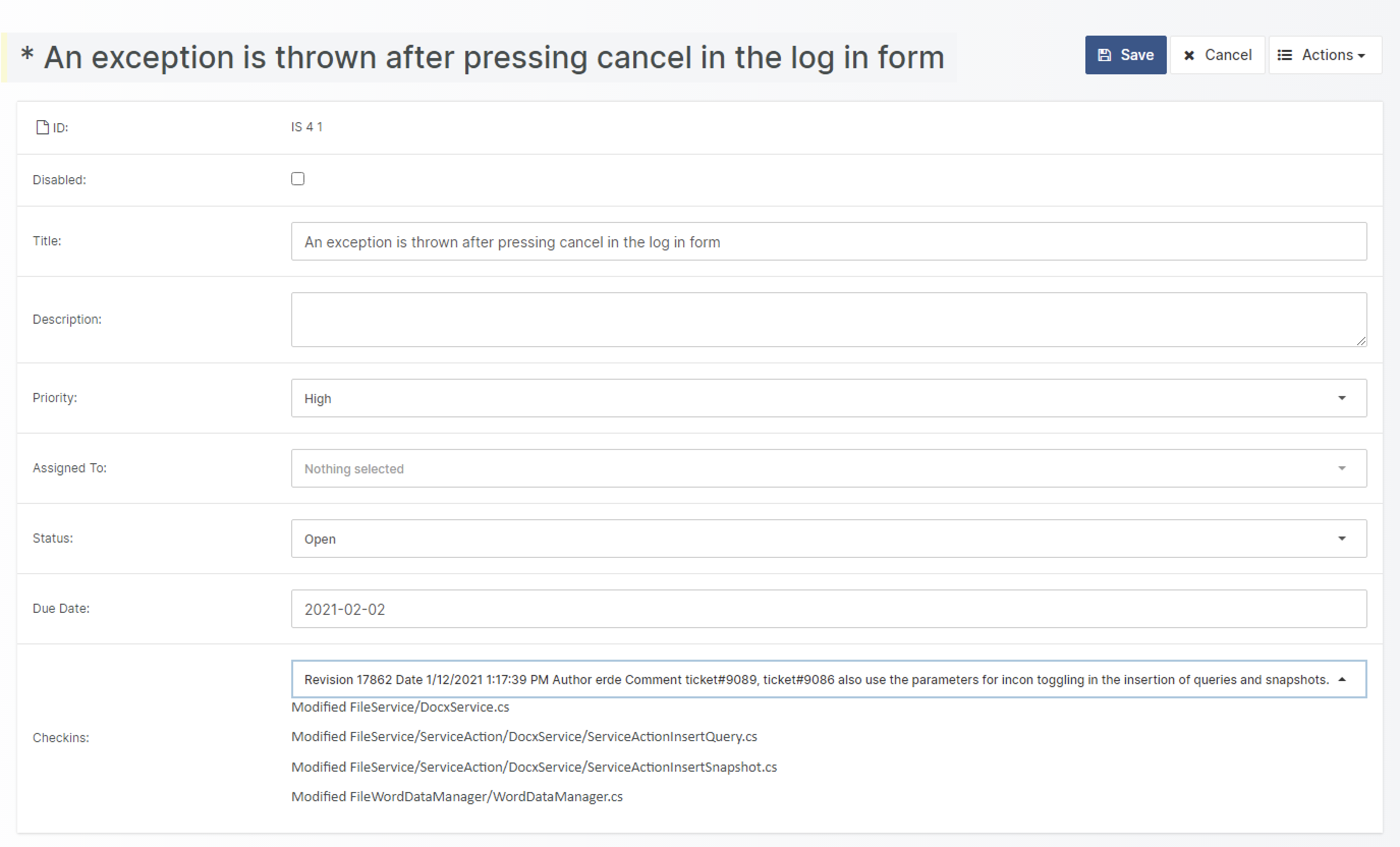
- Change and configuration management (Problem Reports and Change Management)
The Aligned Elements IEC 82304 Configuration can be downloaded here.