EN
Trial Form Test
AE Accordion test
Partners
Partners with us
We know what we do best and what others do better than us. Our Partners make sure that Aligned Elements customers get maximum returns with minimal effort from our system by providing services, trainings, content and on-boarding assistance.
Quality and Regulatory Experts
We team up with an array of Quality and Regulatory experts that can solve your Medical Device Development and Manufacturing issues on your terms.
Keymkr - Keymkr are experts in Compliance Engineering and Product Testing for Medical Devices (MD and IVD). Furthermore, Keymkr develops and market MEDULUS, a software extension for Aligned Elements. MEDULUS makes it possible to create and maintain standards-compliant development documentation throughout the entire life cycle of a medical device using Aligned Elements. Visit Keymkr
Qadvis - QAdvis is a team of expert advisors in compliance, quality and productivity for the MedTech industry. We work with MedTech quality and system management, risk management, compliance, training, interim management and regulatory affairs. Visit Qadvis
ISS - ISS AG provides services for the development of medical devices, their introduction into the market and their maintenance. Typical services include embedded software development, regulatory affairs and clinical evaluation report writing, clinical research, quality management, qualification and validation. Visit ISS
Medidee - Medidee is your partner for medical devices and IVD compliance in Europe and USA. Medidee provides the necessary competencies internally to handle all aspects of your project, from the Design to the Certification. Visit Medidee
Inmedis - Inmedis connects IT and processes. Their services and know how include Regulatory Affairs, Quality Management, QM IT systems, Technical Documentation, Biocompatibility and Computer System Validation. Visit Inmedis
Commala Ltd - Commala offers a service that spans Medical Device Engineering, Regulation and Quality. From the big picture to the devil in the detail; Commala’s hands-on and pragmatic approach to development and engineering is aimed at helping your team get the most out of their assets. Visit Commala
IPP - IPP offers specialized services for medical devices manufacturers in all relevant areas to comply with MDR 2017/745. IPP provides the tool “Easy13485 QMS Software as Service,” to access your process landscape with binding and efficient instructions and the control of ISO 13485 compliance together with a standard-compliant and already filled quality management system. This saves both time and money to get started with your QMS right away. Visit IPP
Usability Experts
For assistance by and access to Human Factors / Usability / IEC 62366 Experts with Aligned Elements experience, reach out to our partners.
Use-Ing - stands for usability engineers and at the same time expresses that our focus is always on representative users and their interaction with various technical products in the respective context of use. Use-Ing successfully supports companies in the medical technology, aeronautical engineering, software engineering, mechanical engineering, mobile machinery and geronto-technology sectors in the human-centered technology development of interactive systems. Visit Use-Ing
Umbrella UX - With our human factors and usability engineering competencies, we support companies in safety-relevant industries to minimize usage-related risks in their products, workflows or processes in order to avoid undesired events such as accidents or product recalls. Visit Umbrella UX
Educational Institution, Clusters and Cooperations
Aligned Elements is used at educational institutions where medical device development is taught.
Hochschule Furtwangen - Aligned Elements is used in the education of medical device at HS Furtwangen, teaching the concepts of Design Control Management. Visit HS Furtwangen
Swiss Medtech - Swiss Medtech is the association of Swiss medical technology, representing around 700 companies. We work closely with our members, informing them of essential developments and supporting them in the event of challenges. Aligned is a member of Swiss Medtech. Visit Swiss Medtech
Health Tech Cluster Switzerland - Health Tech Cluster is a network of manufacturers, suppliers, research and training institutions, service providers and investors in the health technology sector. Aligned is a member of the Health Tech Cluster. Visit the Health Tech Cluster
MDKU - Medical Device Knowledge Units (MDKU) is a non-profit organisation that develops an open source data model for the technical documentation of medical devices to speed up digitalization. Visit MDKU
Want to become a Partner?
Join our partner network to our customers bring world-leading Medical Devices to the market using Aligned Elements
The ISO 14971 risk management tool
The ISO 14971 Risk Management Tool
Stay ISO 14971 compliant and in control
Start your Free Trial now!
Would you like to ...
-
End-to-end Traceability
Quickly reach compliance with ISO 14971 - Application of risk management to medical devices?
-
ISO
Do risk analysis your way and not the way a tool prescribes it?
-
Continuous Traceability
Automatically ensure that your mitigations are implemented?
-
Traceability Tables
Use pre-configured Potential Hazards from ISO 14971?
-
Traceability Tables
Monitor the effect of your mitigations in a pre- and post-mitigation Risk Summary?
-
UNMITIGATED
Be warned if you have incomplete risk coverage or unmitigated risks?
-
LESS
Spend less time on maintaining documents?
Reduce the time spent on Risk Management!
-
End-to-end Traceability
By easily tracing ISO 14971 risk controls / mitigations to your design inputs
-
ISO
By automating checks for not implemented mitigations
-
Continuous Traceability
By checking the completeness of your risk management in seconds
-
Traceability Tables
By re-using mitigations to save time and ensure consistency
-
Traceability Tables
By using configurable (preliminary) hazard analysis (PHA) and FMEA in a single tool
-
PRESS
By creating and updating your risk management files at the press of a button
Start using Aligned Elements!
-
Lower costs
Lower costs for development
-
Minimize work
Minimize the work on development documentation
-
Free up personel
Free up personel for innovation
-
Faster to the market
Bring your Medical Devices to the market faster
The Art Of Medical Device Documentation
The Art Of Medical Device Documentation
-
document less
-
reach compliance fast
-
meet your goals
Get your Personal Online Demo!
And all of this in a single tool?
-
End-to-end Traceability
Manage and Trace your Requirements
-
ISO
Integrate your ISO 14971 compliant Risk Management
-
Continuous Traceability
Effectively manage and document your traceability matrix
-
Traceability Tables
Define and execute Test Protocols
-
Traceability Tables
Perform Design Reviews
No time to evaluate?
If you cannot spare the time to do your own evaluation, let us show Aligned Elements to you in a personal online demo!
This takes about 60 minutes and afterwards you know what Aligned Elements can do for you.
The online demo can also be an efficient kick-start for your own evaluation.
So go ahead and schedule a demo with us!
Complete End-to-end Traceability for your Medical Device
Complete End-to-end Traceability for your Medical Device
-
ISO compatible
-
continuous
-
automated
Request an online presentation now
What you get from Aligned Elements
-
End-to-end Traceability
End-to-end Traceability over all Design Controls
-
ISO
Integrate Traceability with ISO 14971 Risk Assessments
-
Continuous Traceability
Continuous Traceability Quality Monitoring with graphical coverage
-
Traceability Tables
Predefined Medical Device Traceability Tables for IEC 62304
-
Traceability Tables
Automatically generated and synchronized traceability documentation
How Aligned Elements helps your company
-
Lower costs
Lower costs for development
-
Minimize work
Minimize the work on development documentation
-
Free up personel
Free up personel for innovation
-
Faster to the market
Bring your Medical Devices to the market faster
Try free Trial Thank you
Get Started With a Free Trial Today!
What you get for free:
- 30 days free trial
- Access to Web and Windows clients
- Medical Device example project included
- Free technical support
No Credit Card needed!
If you need a trial extension, just let us know.
We received your data and will send you an email with access information.
Don't miss out our follow-up support while you try aligned elements and receive tips on a regular basis!
Need to know more?
If you want assistance to try your own QMS and/or development process in Aligned Elements, we'd be happy to assist you and work out a trial configuration for you.
Seeing your existing development documentation data in Aligned Elements can make it easier for you to assess how the system could assist your organization. We can help you parse and import your existing documentation into a test project.
How do the Aligned Elements Licensing plans work?
Still have questions about Aligned Elements?
Feel free to contact us if you have any questions.
testformular
Customers
Customers
we are proud to be trusted by companies around the world
...and many more
to join our growing list of customers using aligned elements to save time and focus on the interesting part of developing medical devices.
| Company | Instrument Category |
|---|---|
| Abbott | Ablation Catheter |
| Abingdon Health | Diagnostic Rapid Tests |
| AgenaBio | Genotyping |
| AnchorDx | Cancer genomics, genetics and bioinformatics |
| Anvajo | Blood Analysis Instruments |
| AOT | Cold Ablation Robot-guided Laser Osteotome |
| ASSKEA | Aspirators |
| Athena Medical | Medical Trolleys, Screens and Accessories |
| AVA Women | Fertility Tracker |
| Bionano | Optical genome mapping |
| Biotronik | Implants |
| Bambi Medical | Wireless monitoring devices |
| Benchmark Electronics | Wireless Monitoring Systems |
| Biocartis | Molecular Diagnostics |
| Baebies | Medical Equipment Manufacturing |
| BoSonic | Digital Asset Trading |
| BioRad | Diagnostic Instruments |
| BIT Group | Contract Development |
| Brighter | Mobile Health |
| Cavidi | HIV Diagnostics |
| Celon Pharma | Integrated pharmaceutical company |
| Centerline Biomedical | Endovascular Surgery Platform |
| CN Bio Innovation | Organ-on-a-chip |
| CN Systems | Hemodynamics |
| CorTec Neuro | Implantable neurology products |
| Coloplast | Wound care |
| Code Refinery | Medical Device Engineering Consultants |
| CTC Diagnostics | Sample Handling |
| Dawn Clinical, 4s Information Systems | Medical Device Software |
| DATA Corp. | Medical Device Software |
| Decision Sciences Medical | Advanced Image Analysis |
| Diality | Dialysis Machines |
| DJO Global | Orthopedics, sports and health products |
| DNA Electronics | Genomic Diagnostics |
| Dr Langer Medical | Neuromonitoring, Neurostimulation |
| Ellex | Ophtamology |
| Exciscope | X-Ray Microimaging |
| Emblation | Microwave Therapy |
| Endosense | Catheter ablation for the treatment of cardiac arrhythmias |
| Escatec | Medical Device OEM Manufacturer |
| Everex | Medical Device OEM Manufacturer |
| Exini | Image Analysis |
| Economedics | Online Medical Equipment |
| Figur8 | Musculoskeletal health |
| Fluidic | Protein Analysis |
| Fluidnet | Infusion Systems |
| Fraunhofer-Institut für Biomedizinische Technik IBMT | Medical Device Prototyping |
| Geuder | Devices for Ophthalmic surgery |
| Grossenbacher | Control Display, Medical Electronics & EEMS |
| GratXRay | Breast Cancer Imaging |
| Haughton Designs | Medical Device Consultants |
| Hemotune | Magnetic Blood Purification |
| IF Test | Medical Device OEM Manufacturer |
| IH Medical | IVF Solutions |
| Integrated Scientific Services | Medical Device Engineering Consultants |
| Implantica | Implant for prevention of gastroesophageal reflux |
| Inspire Medical Systems | Neurostimulation Device |
| ItoM | Bio- sensing wearable device |
| Konplan | Mechatronic and MedTech |
| KEYMKR | Consultant Service Provider |
| Labitech | Diagnostic Analysers |
| Lifelens | Bio- sensing wearable device |
| Livetec Ingenieurbüro GmbH | OEM Developers |
| Lohmann & Rauscher | Wound Care, Opthalmology, OR Products |
| MBV | Air samplers |
| Medyria | Endovascular Catheters |
| Megin | Brain Imaging |
| MPS AG | Linear bearings and miniature ball screws |
| MinMaxMedical | Surgical robotics and computer-assisted medical interventions |
| MIDI PD | OEM Developers |
| Mycartis | Analysis of Clinical Biomarkers |
| MyoSwiss | Wearable Robotics |
| Mölnlycke Healthcare | Wound Care |
| Nanocellect | Cell sorters |
| NE Scientific LLC | Medical Image Analysis, Modeling, Visualization |
| Nouvag | Medical and Dental Powertools |
| Nuvia Healthtec | Radiodiagnostics, radiotherapy, nuclear medicine and radiopharmacy |
| Oculox Technology | Micro and laser technology |
| Oertli Instruments | Ophtamology Therapy |
| Oculocare | Eye health |
| Organ Assist | Organ Perfusion Systems |
| Orthopediatrics | Pediatric orthopedic instruments |
| Orlight Laser | Surgical lasers |
| Osseointegrations | Custom Implants |
| OstPunkt | Medical Device Engineering Consultants |
| PA Consulting | Medical Device Engineering Consultants |
| Pass | Medical Device Consultants |
| Perimed | Peripheral Vascular Diagnosis |
| Peripal | Connection of dialysis tubings |
| Pjur | Lubrications |
| Portal Instruments | Needle Free Injections |
| Promega | DNA STR Analysis |
| PTW Dosimetry | Radiation Therapy |
| QuantumDX | Molecular Diagnostic Systems |
| Qiagen | Molecular Diagnostics |
| RegenLife | Neurotech and Photomedicine |
| Remp | Sample Management |
| Rotarex S.A. | High pressure valves, tube fittings and pressure regulators |
| Real Heart AB | Heart and Lung machine |
| ReinVAD | Modern Cardiac Support |
| SALTS | Urostomy and Stoma care products |
| Scanco Medical | CT Scanners |
| Schaer Proton | Proton Therapy |
| Sony Mobile Communications | Health Tech |
| Stilla | PCR Systems |
| St Jude Medical | Implantable Heart Pump |
| Storz Medical | Shock wave therapy |
| Super Computing Systems | Medical Device Engineering Consultants |
| T2Biosystems | Blood test Instruments |
| Tecan | Immunodiagnostics, Molecular Diagnostics |
| Thermo Scientific | Tissue processing for anatomical pathology |
| Thermotek | Thermal Therapy Solutions |
| Thoratec (Abbott) | Heart Pumps |
| TMSi | Measurement Systems for electrophysiological parameters |
| Universitätsklinikum RWTH Aachen | Medical Modelling |
| Varian | Brachytherapy |
| Vectura | Inhalation Devices |
| Vetter Pharma | Aseptically pre-filled Application Systems |
| Vision Gate | Software for 3-Dimensional Cell Imaging |
| Volpi | Optoelectronic solutions |
| VOSS Healthcare | Electronically assisted walkers |
| Xvivo Perfusion | Transplantation Systems |
| Ziemer Ophthalmic Systems | Ophthalmic Systems |
Want to know more?
Try aligned elements for free and estimate your time saved.
Miscellaneous Support FAQ
Miscellaneous Support FAQ
Extensions
Accelerate your development with Free Extensions
Enhance Aligned Elements with over 50 available Extensions
Aligned Element Free Extensions
Explore our preconfigured extensions, made available to complete your Technical Documentation faster. Extensions are free add-ons to Aligned Elements that can be downloaded and integrated into your Aligned Elements configuration/projects.
We have made these extensions available for download to speed up your work. Use them to save time, increase transparency or improve the quality of your Design History File. Contact our support for more information on how to deploy and use these extensions.
Word Support FAQ
Word Support FAQ
Documentation
Download Resource Library
Download and benefit from Design Control Management Best Practices and Lessons drawn from hundreds of Medical Device Development Cases.
White Papers
User Manuals
More Best Practices and Lessons Learned?
Sign up with us and be the first one to get new resources and blog posts on Best Practices, Lessons Learned and implications of Regulatory updates.
Database Support FAQ
Database Support FAQ
System Requirements
System Requirements
Windows Client
Recommended Requirements
| RAM | 4 GB |
| Hard Drive | 2 GB Free Space |
| Operating System | Windows 7, Windows 8, Windows 10, Windows 11 |
| MS Word | Word 2013 or later (32-bit or 64-bit) |
Web Client
Recommended Requirements
| Browser | Currently stable release of Edge, Edge Chromium, Google Chrome, Firefox or Safari |
| MS Word | Standalone Word 2019 or later (32-bit or 64-bit) or Word 2013 or later with Office 365 |
Server Requirements
Recommended Requirements
| Processor | 2 cores |
| RAM | 8 GB |
| Hard Drive | 250 GB |
| Operating System | Windows Server 2012 or higher |
| Database Server | Aligned Elements uses Microsoft SQL Server for persistence. |
| SQL Editions | SQL Server Enterprise, SQL Server Standard, SQL Server Web and SQL Server Express with Advanced Services are supported. |
| SQL Versions | SQL Server versions 2005, 2008, 2012, 2014, 2016, 2017, 2019 and 2022 are supported. |
Web Server
A prerequisite for the Aligned Elements Web Client
| Web Server | Aligned Elements uses Microsoft IIS Web Server. |
| Version | IIS 8 or higher |
Request a live demo
Request a live demo
Explain the process
here you can add some introduction regarding the form. People might rather submit this form if they know what to expect.
Try free Trial
Get Started With a Free Trial Today!
What you get for free:
- 30 days free trial
- Access to Web and Windows clients
- Medical Device example project included
- Free technical support
No Credit Card needed!
If you need a trial extension, just let us know.
Need to know more?
If you want assistance to try your own QMS and/or development process in Aligned Elements, we'd be happy to assist you and work out a trial configuration for you.
Seeing your existing development documentation data in Aligned Elements can make it easier for you to assess how the system could assist your organization. We can help you parse and import your existing documentation into a test project.
How do the Aligned Elements Licensing plans work?
Still have questions about Aligned Elements?
Feel free to contact us if you have any questions.
Pricing
Pricing Plans
Start a free 30-days trial today!
Find out more about license types, deployment options, discounts and value packages below!
Node-locked
License tied to a single computer
1300 €
yearly fee
Dedicated to a particular computer
For intensive use
Includes free upgrades and support.
Floating
Popular
Share your License on as many devices you like
2400 €
yearly fee
Shared on any number of computers
For flexible use
Includes free upgrades and support.
Reviewer
View-license for Inspections and Approvals
750 €
yearly fee
Floating viewer licenses
For reading, reviewing and signing
Min. Quantity: 5
Only for Web Client
Team Cost Examples
Floating licenses are great for users that occasionally work on Aligned Elements whereas Node-locked licenses are the obvious choice for dedicated users.
Since floating licenses are shared among several team members, the actual team cost decreases as the team size increases.
For advice on best possible license mix, contact us and request a quote. For starting your Free Trial of Aligned Elements, continue here.
-
Small Team
80 € / month
per user cost
5 users to share
2 floating licenses -
Medium Team
62 € / month
per user cost
20 users to share
5 floating licenses2 users set up with
node-locked licenses
Volume Discounts
We offer tier-based volume discounts starting from the 6th license. The discount per tier applies on the licenses in that tier.
Contact our sales department for any questions on our volume discounts.
| Nr. of Licenses | Discount | |
|---|---|---|
| 6-10 | 6-10 | 5% |
| 11-20 | 11-20 | 10% |
| 20+ | 20+ | 15% |
Aligned Elements Hosted Saas Solution
If our On-Premise or private Cloud deployment options seems like too much hassle, Aligned Elements as Hosted solution is the perfect substitute. The same license types as listed above are used to when access in the Hosted Solution with the additional condition of a minimum number of active licenses required. Aligned Elements as Hosted Solution allows the user to access our Cloud-based repository using the Aligned Elements Web Client.
The Aligned Elements as Hosted Solution Service Level agreement details are listed here.
Named
Aligned Elements Saas with
named licenses
130 € / license
monthly cost
(based on yearly
fee of 1560 €)
Mimimum 10 licenses
Includes free updates, monitoring and support.
Floating
Popular
Aligned Elements Saas
with floating licenses
250 € / license
monthly cost
(based on yearly
fee of 3000 €)
Mimimum 5 licenses
Includes free updates, monitoring and support.
Reviewer
65 € / license
monthly cost
(based on yearly
fee of 780 €)
Mimimum 5 licenses
For reading, reviewing and signing.
Additional Packages
Getting up and running fast is important for most of our customers. To provide best possible fit between Aligned Elements and your existing organization, we offer the following add-ons:
| Configuration Support | 3500 € | Aligning with QMSWe configure Aligned Elements according to your quality management system for seamless integration with your existing QMS processes and templates. |
| On-Site User Training | 2500 € | On-site User TrainingFull-day training of up to 12 of your employees on your company site. The training warrents a certificate of completion if the final test is passed. |
| Online User Training | 1200 € | Online User TrainingFour two-hour online sessions for 12 users. The training warrants a certificate of completion if the final test is passed. |
| Electronic Signatures | 2000 € | Electronic SignaturesAdd 21 CFR Part 11 compliant electronic / digital signature capabilities to your Document Management. Works with and without digital certificates. |
| Integration with External System | 2000 € | Integration with External SystemIntegrate Aligned Elements with Jira, TFS, Enterprise Architect, GitHub and many other system to get the most out of your existing infrastructure. |
| Integration with Automated SW Tests | 2000 € | Integration with Automated SW VerificationLeverage existing automated software tests in your traceability. Integrate Aligned Elements into your continuous build chain and automatically export results from your automated software tests into Aligned Elements. |
| Consulting Services | Deployment, Quick Start and Validation ServicesIf you need further help, we assist you with tasks such as:
|
Request a quote today!
Ask us for a tailored pricing quote!
Home
The faster way to medical devices compliance
Worldclass Design Control Management will accelerate your Technical File / Design History File completion
Core Features
-
Requirements Management
Manage, review and release requirements during the entire Lifeycle. Any changes made are under strict version control with integrated change impact analysis.
-
ISO 14971 Risk Management
Conduct top-down (Preliminary Hazard Analysis) and bottom-up (FMEA) ISO 14971 compliant risk assessments, tightly integrated in the traceability landscape.
-
End to End traceability
Intuitive management of traces, combined with a clear visualization of the traceability status eliminates compliant problems and consistency issues.
-
Verification & Validation
Create, manage and run automated and manual test cases and monitor the progress. Seamlessly integrate with your own bug tracking systems.
-
Document Management
With the industry's most powerful and flexible Word integration in your hands, digitize your Technical File / DHF document management.
-
Analytics and Visualization
Automatically scan and monitor your project to detect and visualize gaps and trends in the consistency and completeness of your DHF.
-
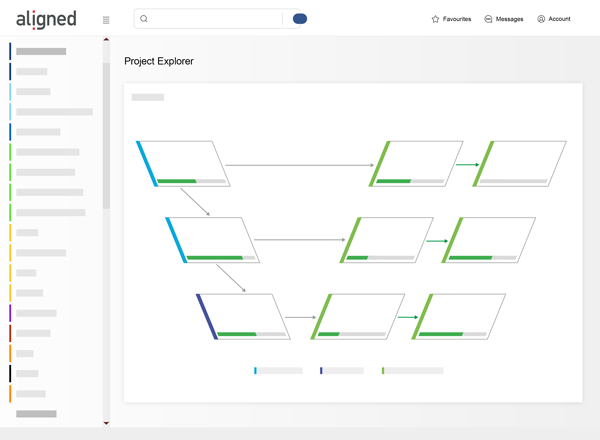
Develop Faster
Is your team held up by documentation work when it should innovate state-of-the-art medical devices?
Medical Device companies report that up to 30% of the total development effort is spent on documentation activities. -
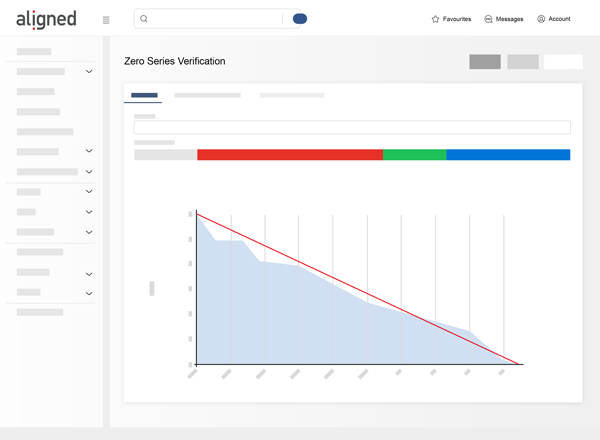
Unmatched Transparency
Aligned Elements integrates and traces all Design Control Items in your application lifecycle into one single application.
Eliminate manual merging and mapping across separate systems and rely on automatic consistency monitoring to drive out quality errors.
-
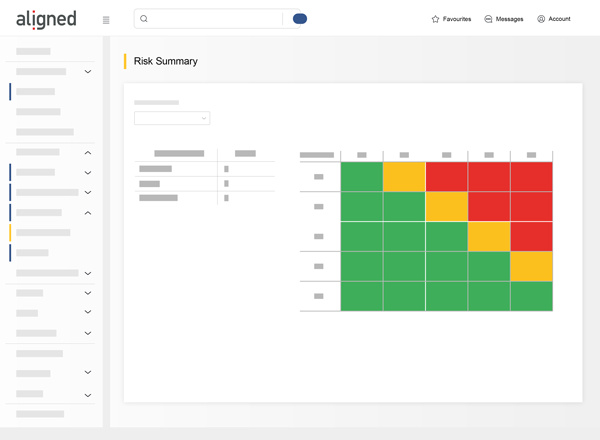
Fast track to regulatory approval
Built for Medical Device development from the ground up, industry regulations and standards are supported out-of-the-box.
Risk Assessments, Design Reviews, DHF Index and Regulatory check lists addresses the special needs of medical device development.
Accelerate your journey to CE Mark and FDA approval
Try aligned elements 30 days for free!
What our customers say
-
Aligned Element have made life easy working with requirement/verification including traceability. We could easily make a structure suitable for us, for all our different products and their specifications.
Anna Norlander, Senior Project Manager, Perimed AB
-
We benefitted by being able to create risk analyses for various product groups quite quickly and easily. A big advantage was that we had to worry less about the form.
Andrea Paulus, Customer Service, Product & QM Support, pjur group Luxembourg
-
Aligned Elements supports a structured way to, starting from requirements, create a traceable set of documentation through the whole lifecycle.
Claes Nilsson, Master System Engineer, Sony Network Communications Europe
-
Thanks to the "linked projects" feature we are able to reduce the duplication of module documentation to a minimum.
Christoph Karthaus, R&D Manager, Tecan Switzerland
-
I can sincerely recommend Aligned Elements to any Medical Device company looking for a single tool to manage requirements, specifications, risks and design reviews.
Victor Steinacher, Project Manager, Levitronix GmbH
-
Amazing to see how adaptable AE is to our development process and risk management process, giving us the ability to work swiftly without any unnecessary constraints!
Jaimin Patel, Regulatory Affairs Manager & Risk Manager
Biotronik connects the dots
user story
New Customer Story
Streamlined Design Controls at Biotronik Vascular Intervention
From duplicated work, manual tracking and the looming risks of documentation inconsistencies to integrated, comprehensible Design Control Management.
Read more about how Biotronik streamlined their Design Control Management using Aligned Elements.
Medical Device Standards and Regulations
Aligned Elements capabilities helps you to stay in compliance with standards and regulations essential to medical device development.
We continously monitor the regulatory landscape to addresses your emerging compliance challenges in upcoming releases.
-
ISO 13485:2016
Built to fulfill design control and change requirements as defined in ISO 13485:2016.
-
ISO 14971:2019
Perform medical device compliant risk assessments in our highly configurable environment.
-
IEC 62304:2015
Use our pre-defined IEC 62304 template set to document and develop medical device software.
-
FDA QSR 820
Design control management capabilities in compliance with the Quality System Regulations (QSR).
-
EU MDR/IVDR
Download, import and kick-start with our pre-configured GSPRs from the EU MDR and IVDR Annex I.
-
IEC 60601-1
A smoother and faster IEC 60606-1 experience with our importable IEC/ISO 60601-1 Checklist.
Why Aligned Elements accelerates your development
-
Reduce time spent on documentation
Is your team held up by documentation work when it should innovate state-of-the-art medical devices?
Then you are not alone.
Medical Device companies report that up to 30% of the total development effort is spent on documentation activities. By spending less time on regulatory documentation, you can free up valuable resources for innovation.
-
Eliminate Design Control errors
By tracking every change, Aligned Elements provides a complete and chronological audit trail of the entire Technical File.
Automatic change control is part of the regular workflow, ensuring a smooth daily use without obstructing the normal operations.
Real-time assessments on the consistency and completeness of the DHF content uncovers any faults and gaps, well in time before the auditor does.
-
100% tailored to your QMS
You have built your QMS to provide maximum efficiency and compliance to your unqie situation.
Therefore, we configure Aligned Elements to each customer's unique QMS, development process and document templates to bring out all the value that has been carefully designed into your unique way of working.
We believe that Aligned Elements shall adapt to you, not the other way around.
-
For Medical Device Developers by Medical Device Experts
Aligned Elements is constructed with the medical device regulations in mind from ground up.
It is designed by Medical Device experts, with decades of experience from real-world medical device development.
These experts constantly update and refine Aligned Elements in response to emerging regulatory challenges with the ultimate goal to reduce your effort and increase the quality of your output.
-
Integrated and connected
Developing medical devices is a complex mission involving a number of engineering disciplines, organizational units and, of course, software systems.
Aligned Elements integrates into your IT landscape to leverage your existing data in your existing systems such as Jira, Azure DevOps, GitHub, Enterprise Architect and many more.
-
Cloud, Hosted or On-Premise
Whether On-premise, in your Cloud or as Hosted Saas, we are flexible.
You decide on the best Aligned Elements deployment option for your company.
We support you with technical assistance in all deployment cases.
Integrates with your existing tool chain



