We get it. You just want to innovate medical devices.
We know you want to focus on making the world's best medical devices.
Not filling out forms.
Or man-handle Excel trace tables.
Or slowly wither away in endless review meetings.
We loved creating medical devices. It's exciting, it's innovative, and it feels good to know we're helping people's health.
But, boy, the Technical Documentation...
We had top-notch tools for engineering, but when it came to documentation, we were stuck with Word and Excel.
And let's be honest, the results weren't great. Auditors kept finding mistakes, no matter how many times we checked our work.
That's why we decided to take action.
In 2006, we set out to create Aligned Elements — a solution designed to make Technical File creation more efficient and free from errors.
Since then, we've continuously refined our approach based on feedback from our clients, ensuring that our tools meet their evolving needs as Medical Device Manufacturers.
At Aligned, we're on a mission to streamline the way Technical Documentation is handled in the medical device industry, ensuring compliance with industry standards such as FDA QSR 820 and EU MDR/IVDR. Founded by a team of experienced medical device developers, we understand the challenges of medical device Technical Documentation first-hand.
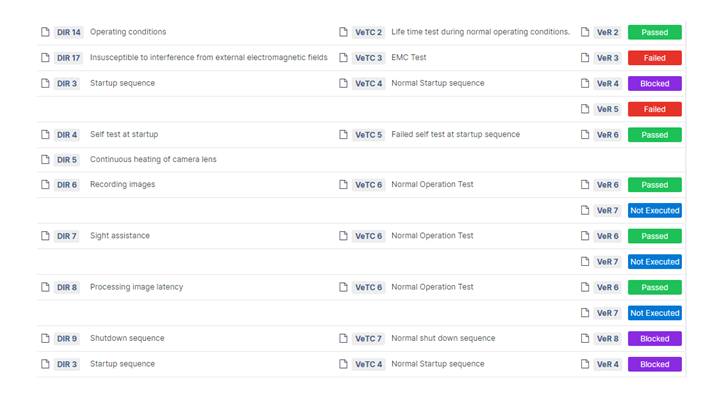
Our goal is simple: to help you spend less time on paperwork and more time on what matters most — developing groundbreaking medical devices. With Aligned Elements, you can navigate regulatory requirements with ease, confident that your documentation is accurate and compliant.
So, let's work together to simplify technical documentation and unleash your full potential for innovation.
At Aligned, we're here to support you every step of the way!